Brexpiprazole | Rexulti
REXULTI is an atypical antipsychotic indicated for:
- Use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD) (1,14.1)
- Treatment of schizophrenia (1,14.2)
Moderate to Severe Hepatic Impairment (Child-Pugh score ≥7): Maximum recommended dosage is 2 mg once daily for patients with MDD and 3 mg once daily for patients with schizophrenia (2.3)
Moderate, Severe or End-Stage Renal Impairment (CLcr<60 mL /minute): Maximum recommended dosage is 2 mg once daily for patients with MDD and 3 mg once daily for patients with schizophrenia (2.4)
Known CYP2D6 Poor Metabolizers: Reduce the usual dosage by half (2.5)
Dosage Forms and Strengths: Tablets: 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg, and 4 mg (3)
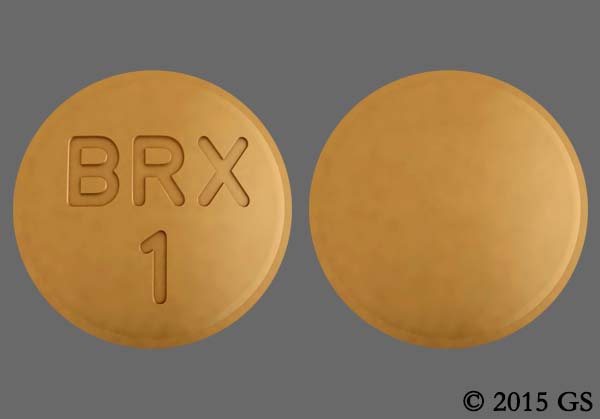
—–INDICATIONS AND USAGE—–
- See description above.
—–DOSAGE AND ADMINISTRATION—–
- See description above.
—–CONTRAINDICATIONS—–
- Known hypersensitivity to REXULTI or any of its components (4)
—–WARNINGS & PRECAUTIONS—–
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g. stroke, transient ischemic attack) (5.3)
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation and close monitoring (5.4)
- Tardive Dyskinesia: Discontinue if clinically appropriate (5.5)
- Metabolic Changes: Monitor for hyperglycemia/diabetes mellitus, dyslipidemia and weight gain (5.6)
- Leukopenia, Neutropenia, and Agranulocytosis: Perform complete blood counts (CBC) in patients with pre-existing low white blood cell count (WBC) or history of leukopenia or neutropenia. Consider discontinuing REXULTI if a clinically significant decline in WBC occurs in absence of other causative factors (5.7)
- Orthostatic Hypotension and Syncope: Monitor heart rate and blood pressure and warn patients with known cardiovascular or cerebrovascular disease, and risk of dehydration or syncope (5.8)
- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower the seizure threshold (5.9)
—–ADVERSE REACTIONS—–
Most common adverse reactions were (6.1):
- MDD: Weight increased and akathisia (≥5% and at least twice the rate for placebo)
- Schizophrenia: Weight increased (≥4% and at least twice the rate for placebo)
—–USE IN SPECIFIC POPUALATIONS—–
Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure (8.1)

